Protein-coding gene in humans
| GDI1 |
|---|
 |
| Identifiers |
|---|
| Aliases | GDI1, 1A, GDIL, MRX41, MRX48, OPHN2, RABGD1A, RABGDIA, XAP-4, GDP dissociation inhibitor 1, XLID41 |
|---|
| External IDs | OMIM: 300104 MGI: 99846 HomoloGene: 37487 GeneCards: GDI1 |
|---|
| Gene location (Human) |
|---|
 | | Chr. | X chromosome (human)[1] |
|---|
| | Band | Xq28 | Start | 154,436,913 bp[1] |
|---|
| End | 154,443,467 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | X chromosome (mouse)[2] |
|---|
| | Band | X A7.3|X 37.97 cM | Start | 73,348,604 bp[2] |
|---|
| End | 73,355,468 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - prefrontal cortex
- Brodmann area 9
- ganglionic eminence
- cingulate gyrus
- parietal lobe
- postcentral gyrus
- nucleus accumbens
- hypothalamus
- amygdala
- anterior pituitary
|
| | Top expressed in | - Region I of hippocampus proper
- cerebellar cortex
- medial dorsal nucleus
- superior frontal gyrus
- paraventricular nucleus of hypothalamus
- subiculum
- dorsomedial hypothalamic nucleus
- ventromedial nucleus
- habenula
- inferior colliculus
|
| | More reference expression data |
|
|---|
| BioGPS | 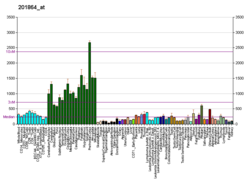 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - Rab GDP-dissociation inhibitor activity
- GTPase activator activity
- protein binding
- GDP-dissociation inhibitor activity
| | Cellular component | - cytoplasm
- cytosol
- Golgi apparatus
- myelin sheath
- midbody
- neuron projection
- axon
- neuronal cell body
- protein-containing complex
| | Biological process | - Rab protein signal transduction
- response to calcium ion
- positive regulation of GTPase activity
- negative regulation of axonogenesis
- negative regulation of protein targeting to membrane
- protein transport
- regulation of catalytic activity
- regulation of small GTPase mediated signal transduction
- signal transduction
- small GTPase mediated signal transduction
- positive regulation of axon extension
- vesicle-mediated transport
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | | |
|---|
| RefSeq (protein) | | |
|---|
| Location (UCSC) | Chr X: 154.44 – 154.44 Mb | Chr X: 73.35 – 73.36 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Rab GDP dissociation inhibitor alpha is a protein that in humans is encoded by the GDI1 gene.[5][6]
Function
GDP dissociation inhibitors are proteins that regulate the GDP-GTP exchange reaction of members of the rab family, small GTP-binding proteins of the ras superfamily, that are involved in vesicular trafficking of molecules between cellular organelles. GDIs slow the rate of dissociation of GDP from rab proteins and release GDP from membrane-bound rabs. GDI1 is expressed primarily in neural and sensory tissues. Mutations in GDI1 have been linked to X-linked nonspecific mental retardation.[7]
Rab GTPases cycles between the cytosolic compartment, where it is bound to a protein called GDI (GDP Dissociation Inhibitor), and the membrane, where it interacts with a receptor, a nucleotide exchange factor, a GAP (GTPase Activating Protein) and probably other factors that link it to the appropriate SNARE. GDI is non-specific with respect to the rab it binds. However, the exchanger, receptor and GAP, are rab specific.
Interactions
GDI1 has been shown to interact with CDC42.[8]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000203879 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000015291 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Bächner D, Sedlacek Z, Korn B, Hameister H, Poustka A (Sep 1995). "Expression patterns of two human genes coding for different rab GDP-dissociation inhibitors (GDIs), extremely conserved proteins involved in cellular transport". Hum. Mol. Genet. 4 (4): 701–8. doi:10.1093/hmg/4.4.701. PMID 7543319.
- ^ Sedlacek Z, Konecki DS, Korn B, Klauck SM, Poustka A (October 1994). "Evolutionary conservation and genomic organization of XAP-4, an Xq28 located gene coding for a human rab GDP-dissociation inhibitor (GDI)". Mammalian Genome. 5 (10): 633–9. doi:10.1007/BF00411459. PMID 7849400. S2CID 6441432.
- ^ "Entrez Gene: GDI1 GDP dissociation inhibitor 1".
- ^ Gibson RM, Wilson-Delfosse AL (Oct 2001). "RhoGDI-binding-defective mutant of Cdc42Hs targets to membranes and activates filopodia formation but does not cycle with the cytosol of mammalian cells". Biochem. J. 359 (Pt 2): 285–94. doi:10.1042/0264-6021:3590285. PMC 1222146. PMID 11583574.
Further reading
- Calinisan V, Gravem D, Chen RP, Brittin S, Mohandas N, Lecomte MC, Gascard P (2006). "New insights into potential functions for the protein 4.1 superfamily of proteins in kidney epithelium". Front. Biosci. 11: 1646–66. doi:10.2741/1911. PMID 16368544. S2CID 26325962.
- Rasmussen HH, van Damme J, Puype M, Gesser B, Celis JE, Vandekerckhove J (1992). "Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes". Electrophoresis. 13 (12): 960–9. doi:10.1002/elps.11501301199. PMID 1286667. S2CID 41855774.
- Matsui Y, Kikuchi A, Araki S, Hata Y, Kondo J, Teranishi Y, Takai Y (1990). "Molecular cloning and characterization of a novel type of regulatory protein (GDI) for smg p25A, a ras p21-like GTP-binding protein". Mol. Cell. Biol. 10 (8): 4116–22. doi:10.1128/mcb.10.8.4116. PMC 360933. PMID 2115118.
- Shisheva A, Südhof TC, Czech MP (1994). "Cloning, characterization, and expression of a novel GDP dissociation inhibitor isoform from skeletal muscle". Mol. Cell. Biol. 14 (5): 3459–68. doi:10.1128/mcb.14.5.3459. PMC 358710. PMID 7513052.
- Nishimura N, Goji J, Nakamura H, Orita S, Takai Y, Sano K (1995). "Cloning of a brain-type isoform of human Rab GDI and its expression in human neuroblastoma cell lines and tumor specimens". Cancer Res. 55 (22): 5445–50. PMID 7585614.
- Shapiro AD, Pfeffer SR (1995). "Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides". J. Biol. Chem. 270 (19): 11085–90. doi:10.1074/jbc.270.19.11085. PMID 7744738.
- Beranger F, Cadwallader K, Porfiri E, Powers S, Evans T, de Gunzburg J, Hancock JF (1994). "Determination of structural requirements for the interaction of Rab6 with RabGDI and Rab geranylgeranyltransferase". J. Biol. Chem. 269 (18): 13637–43. doi:10.1016/S0021-9258(17)36877-1. PMID 8175798.
- Sedlacek Z, Korn B, Konecki DS, Siebenhaar R, Coy JF, Kioschis P, Poustka A (1993). "Construction of a transcription map of a 300 kb region around the human G6PD locus by direct cDNA selection". Hum. Mol. Genet. 2 (11): 1865–9. doi:10.1093/hmg/2.11.1865. PMID 8281148.
- Hancock JF, Hall A (1993). "A novel role for RhoGDI as an inhibitor of GAP proteins". EMBO J. 12 (5): 1915–21. doi:10.1002/j.1460-2075.1993.tb05840.x. PMC 413412. PMID 8491184.
- Chen EY, Zollo M, Mazzarella R, Ciccodicola A, Chen CN, Zuo L, Heiner C, Burough F, Repetto M, Schlessinger D, D'Urso M (1996). "Long-range sequence analysis in Xq28: thirteen known and six candidate genes in 219.4 kb of high GC DNA between the RCP/GCP and G6PD loci". Hum. Mol. Genet. 5 (5): 659–68. doi:10.1093/hmg/5.5.659. PMID 8733135.
- Hamel BC, Kremer H, Wesby-van Swaay E, van den Helm B, Smits AP, Oostra BA, Ropers HH, Mariman EC (1996). "A gene for nonspecific X-linked mental retardation (MRX41) is located in the distal segment of Xq28". Am. J. Med. Genet. 64 (1): 131–3. doi:10.1002/(SICI)1096-8628(19960712)64:1<131::AID-AJMG22>3.0.CO;2-N. hdl:2066/23576. PMID 8826463.
- Wilson AL, Sheridan KM, Erdman RA, Maltese WA (1996). "Prenylation of a Rab1B mutant with altered GTPase activity is impaired in cell-free systems but not in intact mammalian cells". Biochem. J. 318 (3): 1007–14. doi:10.1042/bj3181007. PMC 1217717. PMID 8836150.
- des Portes V, Billuart P, Carrié A, Bachner L, Bienvenu T, Vinet MC, Beldjord C, Ponsot G, Kahn A, Boué J, Chelly J (1997). "A gene for dominant nonspecific X-linked mental retardation is located in Xq28". Am. J. Hum. Genet. 60 (4): 903–9. PMC 1712488. PMID 9106537.
- Gorvel JP, Chang TC, Boretto J, Azuma T, Chavrier P (1998). "Differential properties of D4/LyGDI versus RhoGDI: phosphorylation and rho GTPase selectivity". FEBS Lett. 422 (2): 269–73. doi:10.1016/S0014-5793(98)00020-9. PMID 9490022. S2CID 10817327.
- D'Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, Gedeon AK, Oostra B, Wu SK, Tandon A, Valtorta F, Balch WE, Chelly J, Toniolo D (1998). "Mutations in GDI1 are responsible for X-linked non-specific mental retardation". Nat. Genet. 19 (2): 134–9. doi:10.1038/487. PMID 9620768. S2CID 21043350.
- Bienvenu T, des Portes V, Saint Martin A, McDonell N, Billuart P, Carrié A, Vinet MC, Couvert P, Toniolo D, Ropers HH, Moraine C, van Bokhoven H, Fryns JP, Kahn A, Beldjord C, Chelly J (1998). "Non-specific X-linked semidominant mental retardation by mutations in a Rab GDP-dissociation inhibitor". Hum. Mol. Genet. 7 (8): 1311–5. doi:10.1093/hmg/7.8.1311. PMID 9668174.
- Wan M, Francke U (1998). "Evaluation of two X chromosomal candidate genes for Rett syndrome: glutamate dehydrogenase-2 (GLUD2) and rab GDP-dissociation inhibitor (GDI1)". Am. J. Med. Genet. 78 (2): 169–72. doi:10.1002/(SICI)1096-8628(19980630)78:2<169::AID-AJMG14>3.0.CO;2-L. PMID 9674910.
- Hutt DM, Da-Silva LF, Chang LH, Prosser DC, Ngsee JK (2000). "PRA1 inhibits the extraction of membrane-bound rab GTPase by GDI1". J. Biol. Chem. 275 (24): 18511–9. doi:10.1074/jbc.M909309199. PMID 10751420.
PDB gallery
-
1d5t: GUANINE NUCLEOTIDE DISSOCIATION INHIBITOR, ALPHA-ISOFORM -
1gnd: GUANINE NUCLEOTIDE DISSOCIATION INHIBITOR, ALPHA-ISOFORM -
1lv0: Crystal structure of the Rab effector guanine nucleotide dissociation inhibitor (GDI) in complex with a geranylgeranyl (GG) peptide |
 | This article on a gene on the human X chromosome and/or its associated protein is a stub. You can help Wikipedia by expanding it. |

 1d5t: GUANINE NUCLEOTIDE DISSOCIATION INHIBITOR, ALPHA-ISOFORM
1d5t: GUANINE NUCLEOTIDE DISSOCIATION INHIBITOR, ALPHA-ISOFORM 1gnd: GUANINE NUCLEOTIDE DISSOCIATION INHIBITOR, ALPHA-ISOFORM
1gnd: GUANINE NUCLEOTIDE DISSOCIATION INHIBITOR, ALPHA-ISOFORM 1lv0: Crystal structure of the Rab effector guanine nucleotide dissociation inhibitor (GDI) in complex with a geranylgeranyl (GG) peptide
1lv0: Crystal structure of the Rab effector guanine nucleotide dissociation inhibitor (GDI) in complex with a geranylgeranyl (GG) peptide



















