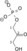| Fosfopiruvat hidrataza |
| Identifikatori |
| EC broj | 4.2.1.11 |
| CAS broj | 9014-08-8 |
| IntEnz | IntEnz view |
| BRENDA | BRENDA entry |
| ExPASy | NiceZyme view |
| KEGG | KEGG entry |
| MetaCyc | metabolic pathway |
| PRIAM | profile |
| PDB | RCSB PDB PDBe PDBj PDBsum |
| Ontologija gena | AmiGO / EGO |
| Pretraga | | PMC | articles | | PubMed | articles | | NCBI Protein | search | |
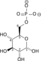
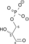
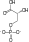
U enzimologiji, fosfopiruvat hidrataza (EC 4.2.1.11) je enzim koji katalizuje hemijsku reakciju[1][2][3]
- 2-fosfo-D-glicerat
 fosfoenolpiruvat + H2O
fosfoenolpiruvat + H2O
Ovaj enzim ima jedan supstrat, 2-fosfo-D-glicerat, i dva produkta, fosfoenolpiruvat i H2O.
Ovaj enzim pripada familiji lijaza, specifično hidrolijaza, koji presecaju ugljenik-kiseonik veze. Sistematsko ime ove klase enzima je 2-fosfo-D-glicerat hidrolijaza (fosfoenolpiruvat formirajuća). Druga imena u čestoj upotrebi su: enolaza, 2-fosfoglicerat dehidrataza, 14-3-2-protein, specifična enolaza nervnog sistema, fosfoenolpiruvat hidrataza, 2-fosfoglicerat dehidrataza, 2-fosfoglicerinska dehidrataza, 2-fosfoglicerat enolaza i gama-enolaza. Ovaj enzim učestvuje u glikolizi/glukoneogenezi. Jedan od poznatih inhibitora je fosfonoacetohidroksamat. Ljudski gen za ovaj enzim je ENO2.
Strukturne studije
Neke od rešenih struktura enzima ove klase su: 1E9I, 1EBG, 1EBH, 1ELS, 1IYX, 1L8P, 1NEL, 1OEP, 1ONE, 1P43, 1P48, 1PDY, 1PDZ, 1TE6, 1W6T, 2AKM, 2AKZ, 2AL1, 2AL2, 2FYM, 2ONE, 2PA6, 3ENL, 4ENL, 5ENL, 6ENL, and 7ENL.
Reference
- ↑ Pancholi V (June 2001). „Multifunctional α-enolase: its role in diseases”. Cell Mol Life Sci. 58 (7): 902–20. DOI:10.1007/PL00000910. PMID 11497239.
- ↑ Hoorn RK, Flickweert JP, Staal GE (1974). „Purification and properties of enolase of human erythroctyes”. Int J Biochem 5 (11–12): 845–52. DOI:10.1016/0020-711X(74)90119-0.
- ↑ Lohman K & Meyerhof O (1934) Über die enzymatische umwandlung von phosphoglyzerinsäure in brenztraubensäure und phosphorsäure (Enzymatic transformation of phosphoglyceric acid into pyruvic and phosphoric acid). Biochem Z 273, 60–72.
Literatura
- Nicholas C. Price, Lewis Stevens (1999). Fundamentals of Enzymology: The Cell and Molecular Biology of Catalytic Proteins (Third izd.). USA: Oxford University Press. ISBN 019850229X.
- Eric J. Toone (2006). Advances in Enzymology and Related Areas of Molecular Biology, Protein Evolution (Volume 75 izd.). Wiley-Interscience. ISBN 0471205036.
- Branden C, Tooze J.. Introduction to Protein Structure. New York, NY: Garland Publishing. ISBN: 0-8153-2305-0.
- Irwin H. Segel. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems (Book 44 izd.). Wiley Classics Library. ISBN 0471303097.
- Robert A. Copeland (2013). Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists (2nd izd.). Wiley-Interscience. ISBN 111848813X.
- Gerhard Michal, Dietmar Schomburg (2012). Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology (2nd izd.). Wiley. ISBN 0470146842.
- Holt A, Wold F (1961). „The isolation and characterization of rabbit muscle enolase”. J. Biol. Chem. 236: 3227-31. PMID 13908561.
- Boyer, P.D., Lardy, H. and Myrback, K. (Eds.), The Enzymes, 2nd ed., vol. 5, Academic Press, New York, 1961, p. 471-494.
- Westhead EW, McLain G (1964). „A Purification of Brewers' and Bakers' Yeast Enolase Yielding a Single Active Component”. J. Biol. Chem. 239: 2464-8. PMID 14235523.
|
| mt, k, c/g/r/p/y/i, f/h/s/l/o/e, a/u, n, m | k, cgrp/y/i, f/h/s/l/o/e, au, n, m, epon | m (A16/C10), i (k, c/g/r/p/y/i, f/h/s/o/e, a/u, n, m) |
|
|
|
|---|
| Teme | |
|---|
| Tipovi | |
|---|
B enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 |