 α-Sb2O4
| |
 | |
| Names | |
|---|---|
| IUPAC name
antimony(III,V) oxide
| |
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | 100.014.161 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| SbO2; Sb2O4 | |
| Molar mass | 153.7588; 307.5176 g/mol |
| Appearance | white solid |
| Density | 6.64 g/cm3 (orthorhombic form) [1] |
| Melting point | > 930 °C (1,710 °F; 1,200 K) (decomposes) |
| Boiling point | decomposes |
| insoluble | |
Refractive index (nD)
|
2.0 |
| Structure | |
| orthorhombic | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 mg/m3 (as Sb)[2] |
REL (Recommended)
|
TWA 0.5 mg/m3 (as Sb)[2] |
| Related compounds | |
Related compounds
|
Antimony trioxide Antimony pentoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Antimony tetroxide is an inorganic compound with the formula Sb2O4. This material, which exists as the mineral cervantite,[3] is white but reversibly yellows upon heating. The material, with empirical formula SbO2, is called antimony tetroxide to signify the presence of two kinds of Sb centers.[4]

Formation and structure
[edit]The material forms when Sb2O3 is heated in air:[5]
- Sb2O3 + 0.5 O2 → Sb2O4 ΔH = −187 kJ/mol
At 800 °C, antimony(V) oxide loses oxygen to give the same material:
- Sb2O5 → Sb2O4 + 0.5 O2 ΔH = −64 kJ/mol
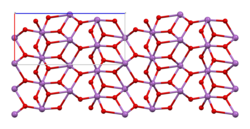
The material is mixed valence, containing both Sb(V) and Sb(III) centers. Two polymorphs are known, one orthorhombic (shown in the infobox) and one monoclinic.[1] Both forms feature octahedral Sb(V) centers arranged in sheets with distorted Sb(III) centers bound to four oxides.
References
[edit]- ^ a b Amador, J.; Puebla, E. Gutierrez; Monge, M. A.; Rasines, I.; Valero, C. Ruiz (1988). "Diantimony Tetraoxides Revisited". Inorganic Chemistry. 27 (8): 1367–1370. doi:10.1021/ic00281a011.
- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Cervantite". Webminerals. Retrieved 2009-06-06.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 576. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
