 | |
 | |
| Names | |
|---|---|
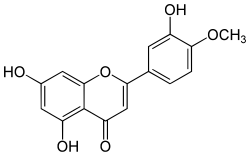
| IUPAC name
3′,5,7-Trihydroxy-4′-methoxyflavone
| |
| Systematic IUPAC name
5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-benzopyran-4-one | |
| Other names
Luteolin 4′-methyl ether
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.539 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O6 | |
| Molar mass | 300.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diosmetin, also known as 5,7,3′-trihydroxy-4′-methoxyflavone, is an O-methylated flavone, a chemical compound that can be found in the Caucasian vetch.[1]
It has been found to act as a weak TrkB receptor agonist.[2]
Glycosides
[edit]Diosmetin is the aglycone of diosmin.
See also
[edit]References
[edit]- ^ Andreeva, O. A.; Ivashev, M. N.; Ozimina, I. I.; Maslikova, G. V. (1998). "Diosmetin glycosides from caucasian vetch: Isolation and study of biological activity". Pharmaceutical Chemistry Journal. 32 (11): 595–597. doi:10.1007/BF02465832. S2CID 21434373.
- ^ Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K (2010). "A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone". Proc. Natl. Acad. Sci. U.S.A. 107 (6): 2687–92. Bibcode:2010PNAS..107.2687J. doi:10.1073/pnas.0913572107. PMC 2823863. PMID 20133810.