 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.855 |
| Chemical and physical data | |
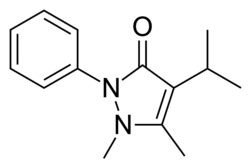
| Formula | C14H18N2O |
| Molar mass | 230.311 g·mol−1 |
| 3D model (JSmol) | |
| |
| |

Propyphenazone (known as isopropylantipyrine in Japan)[1] is a derivative of phenazone[2] with similar analgesic and antipyretic effects. Originally patented in 1931,[3] propyphenazone is marketed as a combination formulation with paracetamol and caffeine for treatment of primary headache disorder.[4]
Serious adverse events
[edit]Case reports have described acute inferior-wall myocardial infarctions characterized by low atrial rhythms[vague] (Kounis syndrome) secondary to propyphenazone use.[5]
Excerpt from WHO comments
[edit]Propyphenazone, a pyrazolone derivative with anti-inflammatory, analgesic and antipyretic activity, was introduced in 1951 for the treatment of rheumatic disorders. As it is structurally related to aminophenazone it has been associated with severe blood dyscrasias. However, it cannot be transformed into potentially carcinogenic nitrosamines and has therefore been widely used as a replacement drug for aminophenazone. In certain countries, products containing propyphenazone have now been restricted in their indications, whereas in others they are still available, sometimes as over-the-counter preparations.[6]
Banned
[edit]Propyphenazone is banned in some countries including Sri Lanka,[7] Malaysia,[7] and Thailand.[7]
Synthesis
[edit]Ethyl 2-isopropylacetoacetate (1) and phenylhydrazine (2) are combined to form the pyrazolone ring in the intermediate (3), which is alkylated with methyl iodide to yield propyphenazone.[8][9]
See also
[edit]References
[edit]- ^ "Isopropylantipyrine". Drugs.com. Retrieved 8 March 2018.
- ^ Göres E, Kossowicz J, Schneider HG (March 2004). "[Propyphenazone. Pharmacology and use]" [Propyphenazone. Pharmacology and use]. Medizinische Monatsschrift für Pharmazeuten (in German). 27 (3): 72–6. PMID 15032249.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 530. ISBN 9783527607495.
- ^ "SC exempts painkiller Saridon from the banned list in India". The Economic Times. February 21, 2019.
- ^ Akyel A, Alsancak Y, Yayla Ç, Sahinarslan A, Özdemir M (May 2011). "Acute inferior myocardial infarction with low atrial rhythm due to propyphenazone: Kounis syndrome". International Journal of Cardiology. 148 (3): 352–3. doi:10.1016/j.ijcard.2010.05.038. PMID 20541820.
- ^ Consolidated List of Products whose Consumption and/or Sale have been Banned, Withdrawn, Severely Restricted or not Approved by Governments, Twelfth Issue (PDF). New York: Department of Economic and Social Affairs of the United Nations Secretariat. 2005. p. 232.
- ^ a b c "Multi-Country Survey On Banned And Restricted Pharmaceuticals". Health Action International Asia Pacific. August 2008. p. 7.
- ^ Sawa, Yoshiro (1937). "Synthese verschiedener Pyrazolonderivate". Yakugaku Zasshi (in German). 57 (11): 953–962. doi:10.1248/yakushi1881.57.11_953.
- ^ "Propyphenazone". Pharmaceutical Substances. Thieme. Retrieved 2024-07-17.
