 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
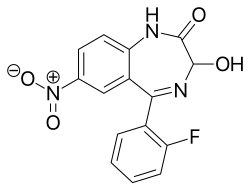
| Formula | C15H10FN3O4 |
| Molar mass | 315.260 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nifoxipam (3-hydroxydesmethylflunitrazepam, DP 370) is a benzodiazepine that is a minor metabolite of flunitrazepam and has been sold online as a designer drug.[1][2][3][4][5][6][7][8][9]
Nifoxipam produces strong tranquillising and sleep-prolonging effects and has much lower toxicity compared to lormetazepam and flunitrazepam in mice.[1]
See also
[edit]References
[edit]- ^ a b EP 0158267, Posselt K, Wagener HH, Gruber K,, "Pharmaceutical composition containing 5-(2-fluorophenyl)-1,3-dihydro-3-hydroxy-7-nitro- or 5-(2-fluorophenyl)-1,3-dihydro-3-hydroxy-1-methyl-7-nitro-2H-1,4-benzodiazepin-2-one and process for their preparation", published 16 October 1985, assigned to Dolorgiet Beteiligungs-GmbH
- ^ "Nifoxipam". New Synthetic Drugs Database. Archived from the original on 2016-11-01. Retrieved 2016-07-08.
- ^ Kilicarslan T, Haining RL, Rettie AE, Busto U, Tyndale RF, Sellers EM (April 2001). "Flunitrazepam metabolism by cytochrome P450S 2C19 and 3A4". Drug Metabolism and Disposition. 29 (4 Pt 1): 460–5. PMID 11259331.
- ^ Moosmann B, King LA, Auwärter V (June 2015). "Designer benzodiazepines: A new challenge". World Psychiatry. 14 (2): 248. doi:10.1002/wps.20236. PMC 4471986. PMID 26043347.
- ^ Kevin Flemen (August 2015). "Drug Facts - Newer Unregulated Drugs" (PDF). KFx. Retrieved 15 August 2015.
- ^ "Nifoxipam". WEDINOS.
- ^ Meyer MR, Bergstrand MP, Helander A, Beck O (May 2016). "Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes". Analytical and Bioanalytical Chemistry. 408 (13): 3571–91. doi:10.1007/s00216-016-9439-6. PMID 27071765. S2CID 25831532.
- ^ Pettersson Bergstrand M, Helander A, Hansson T, Beck O (April 2017). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis. 9 (4): 640–645. doi:10.1002/dta.2003. PMID 27366870.
- ^ Katselou M, Papoutsis I, Nikolaou P, Spiliopoulou C, Athanaselis S (2016). "Metabolites replace the parent drug in the drug arena. The cases of fonazepam and nifoxipam". Forensic Toxicology. 35 (1): 1–10. doi:10.1007/s11419-016-0338-5. PMC 5214877. PMID 28127407.