Mammalian protein found in Homo sapiens
| CACNA1S |
|---|
|
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
|
|
| Identifiers |
|---|
| Aliases | CACNA1S, CACNL1A3, CCHL1A3, Cav1.1, HOKPP, HOKPP1, MHS5, TTPP1, hypoPP, calcium voltage-gated channel subunit alpha1 S, DHPR |
|---|
| External IDs | OMIM: 114208 MGI: 88294 HomoloGene: 37257 GeneCards: CACNA1S |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 1 (human)[1] |
|---|
| | Band | 1q32.1 | Start | 201,039,512 bp[1] |
|---|
| End | 201,112,451 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 1 (mouse)[2] |
|---|
| | Band | 1 E4|1 59.55 cM | Start | 135,980,488 bp[2] |
|---|
| End | 136,047,560 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - triceps brachii muscle
- vastus lateralis muscle
- thoracic diaphragm
- gastrocnemius muscle
- deltoid muscle
- tibialis anterior muscle
- body of tongue
- trabecular bone
- superior surface of tongue
- synovial joint
|
| | Top expressed in | - extensor digitorum longus muscle
- plantaris muscle
- triceps brachii muscle
- ankle
- temporal muscle
- sternocleidomastoid muscle
- quadriceps femoris muscle
- skeletal muscle tissue
- digastric muscle
- tibialis anterior muscle
|
| | More reference expression data |
|
|---|
| BioGPS |  | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - voltage-gated calcium channel activity
- calcium channel activity
- metal ion binding
- voltage-gated ion channel activity
- high voltage-gated calcium channel activity
- ion channel activity
- protein binding
- calmodulin binding
| | Cellular component | - cytoplasm
- voltage-gated calcium channel complex
- integral component of membrane
- membrane
- I band
- T-tubule
- L-type voltage-gated calcium channel complex
- plasma membrane
- sarcolemma
| | Biological process | - muscle contraction
- membrane depolarization during action potential
- regulation of ion transmembrane transport
- ion transport
- calcium ion transmembrane transport
- transmembrane transport
- calcium ion transport
- cellular response to caffeine
- calcium ion import
- cardiac conduction
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | | |
|---|
| RefSeq (protein) | | |
|---|
NP_001074492
NP_055008
NP_001389878 |
|
|---|
| Location (UCSC) | Chr 1: 201.04 – 201.11 Mb | Chr 1: 135.98 – 136.05 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Cav1.1 also known as the calcium channel, voltage-dependent, L type, alpha 1S subunit, (CACNA1S), is a protein which in humans is encoded by the CACNA1S gene.[5] It is also known as CACNL1A3 and the dihydropyridine receptor (DHPR, so named due to the blocking action DHP has on it).
Function
This gene encodes one of the five subunits of the slowly inactivating L-type voltage-dependent calcium channel in skeletal muscle cells. Mutations in this gene have been associated with hypokalemic periodic paralysis, thyrotoxic periodic paralysis and malignant hyperthermia susceptibility.[5]
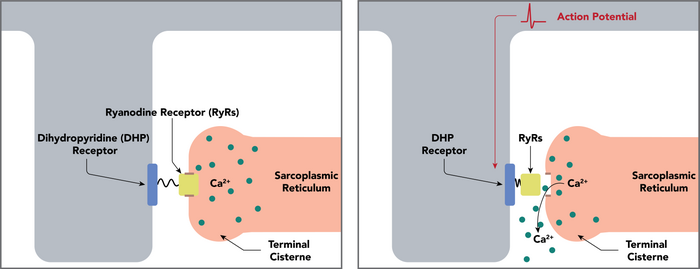
Cav1.1 is a voltage-dependent calcium channel found in the transverse tubule of muscles. In skeletal muscle it associates with the ryanodine receptor RyR1 of the sarcoplasmic reticulum via a mechanical linkage. It senses the voltage change caused by the end-plate potential from nervous stimulation and propagated by sodium channels as action potentials to the T-tubules. It was previously thought that when the muscle depolarises, the calcium channel opens, allowing calcium in and activating RyR1, which mediates much greater calcium release from the sarcoplasmic reticulum. This is the first part of the process of excitation-contraction coupling, which ultimately causes the muscle to contract. Calcium entry through Cav1.1 is not required in skeletal muscle, as it is in cardiac muscle; Cav1.1 undergoes a conformational change which allosterically activates RyR1.[6]
Clinical significance
In hypokalemic periodic paralysis (HOKPP), the voltage sensors in domains 2 and 4 of Cav1.1 are mutated (loss-of-function), reducing the availability of the channel to sense depolarisation, and therefore it cannot activate the ryanodine receptor as efficiently. As a result, the muscle cannot contract very well and the patient is paralysed. The condition is hypokalemic because a low extracellular potassium ion concentration will cause the muscle to repolarise to the resting potential more quickly, so any calcium conductance that does occur cannot be sustained. It becomes more difficult to reach the threshold at which the muscle can contract, and even if this is reached then the muscle is more prone to relaxing. Because of this, the severity would be reduced if potassium ion concentrations are maintained. In contrast, hyperkalemic periodic paralysis refers to gain-of-function mutations in sodium channels that maintain muscle depolarisation and therefore are aggravated by high potassium ion concentrations.[7]
The European Malignant Hyperthermia Group accepts two mutations in CACNA1S as diagnostic for malignant hyperthermia.[8]
Blockers
Cav1.1 is blocked by dihydropyridine.
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000081248 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000026407 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b "Entrez Gene: CACNA1S calcium channel, voltage-dependent, L type, alpha 1S subunit".
- ^ Proenza C, O'Brien J, Nakai J, Mukherjee S, Allen PD, Beam KG (February 2002). "Identification of a region of RyR1 that participates in allosteric coupling with the alpha(1S) (Ca(V)1.1) II-III loop". J. Biol. Chem. 277 (8): 6530–5. doi:10.1074/jbc.M106471200. PMID 11726651.
- ^ Jurkat-Rott K, Lehmann-Horn F (August 2005). "Muscle channelopathies and critical points in functional and genetic studies". J. Clin. Invest. 115 (8): 2000–9. doi:10.1172/JCI25525. PMC 1180551. PMID 16075040.
- ^ "European Malignant Hyperthermia Group: Mutations in RYR1". Archived from the original on 2016-03-21. Retrieved 2015-05-14.
Further reading
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (2005). "International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels". Pharmacol. Rev. 57 (4): 411–25. doi:10.1124/pr.57.4.5. PMID 16382099. S2CID 10386627.
- Rotman EI, De Jongh KS, Florio V, Lai Y, Catterall WA (1992). "Specific phosphorylation of a COOH-terminal site on the full-length form of the alpha 1 subunit of the skeletal muscle calcium channel by cAMP-dependent protein kinase". J. Biol. Chem. 267 (23): 16100–5. doi:10.1016/S0021-9258(18)41972-2. PMID 1322891.
- Röhrkasten A, Meyer HE, Nastainczyk W, Sieber M, Hofmann F (1988). "cAMP-dependent protein kinase rapidly phosphorylates serine- 687 of the skeletal muscle receptor for calcium channel blockers". J. Biol. Chem. 263 (30): 15325–9. doi:10.1016/S0021-9258(19)37591-X. PMID 2844809.
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S (1987). "Primary structure of the receptor for calcium channel blockers from skeletal muscle". Nature. 328 (6128): 313–8. Bibcode:1987Natur.328..313T. doi:10.1038/328313a0. PMID 3037387. S2CID 4325355.
- Hogan K, Powers PA, Gregg RG (1994). "Cloning of the human skeletal muscle alpha 1 subunit of the dihydropyridine-sensitive L-type calcium channel (CACNL1A3)". Genomics. 24 (3): 608–9. doi:10.1006/geno.1994.1677. PMID 7713519.
- Elbaz A, Vale-Santos J, Jurkat-Rott K, Lapie P, Ophoff RA, Bady B, Links TP, Piussan C, Vila A, Monnier N (1995). "Hypokalemic periodic paralysis and the dihydropyridine receptor (CACNL1A3): genotype/phenotype correlations for two predominant mutations and evidence for the absence of a founder effect in 16 caucasian families". Am. J. Hum. Genet. 56 (2): 374–80. PMC 1801148. PMID 7847370.
- Boerman RH, Ophoff RA, Links TP, van Eijk R, Sandkuijl LA, Elbaz A, Vale-Santos JE, Wintzen AR, van Deutekom JC, Isles DE (1995). "Mutation in DHP receptor alpha 1 subunit (CACLN1A3) gene in a Dutch family with hypokalaemic periodic paralysis". J. Med. Genet. 32 (1): 44–7. doi:10.1136/jmg.32.1.44. PMC 1050178. PMID 7897626.
- Gregg RG, Couch F, Hogan K, Powers PA (1993). "Assignment of the human gene for the alpha 1 subunit of the skeletal muscle DHP-sensitive Ca2+ channel (CACNL1A3) to chromosome 1q31-q32". Genomics. 15 (1): 107–12. doi:10.1006/geno.1993.1017. PMID 7916735.
- Jurkat-Rott K, Lehmann-Horn F, Elbaz A, Heine R, Gregg RG, Hogan K, Powers PA, Lapie P, Vale-Santos JE, Weissenbach J (1994). "A calcium channel mutation causing hypokalemic periodic paralysis". Hum. Mol. Genet. 3 (8): 1415–9. doi:10.1093/hmg/3.8.1415. PMID 7987325.
- Ptácek LJ, Tawil R, Griggs RC, Engel AG, Layzer RB, Kwieciński H, McManis PG, Santiago L, Moore M, Fouad G (1994). "Dihydropyridine receptor mutations cause hypokalemic periodic paralysis". Cell. 77 (6): 863–8. doi:10.1016/0092-8674(94)90135-X. PMID 8004673. S2CID 13538157.
- Drouet B, Garcia L, Simon-Chazottes D, Mattei MG, Guénet JL, Schwartz A, Varadi G, Pinçon-Raymond M (1993). "The gene coding for the alpha 1 subunit of the skeletal dihydropyridine receptor (Cchl1a3 = mdg) maps to mouse chromosome 1 and human 1q32". Mamm. Genome. 4 (9): 499–503. doi:10.1007/BF00364784. PMID 8118099. S2CID 1386074.
- Iles DE, Segers B, Olde Weghuis D, Suijkerbuijk R, Mikala G, Schwartz A, Wieringa B (1994). "Refined localization of the alpha 1-subunit of the skeletal muscle L-type voltage-dependent calcium channel (CACNL1A3) to human chromosome 1q32 by in situ hybridization". Genomics. 19 (3): 561–3. doi:10.1006/geno.1994.1106. PMID 8188298.
- O'Brien RO, Taske NL, Hansbro PM, Matthaei KI, Hogan SP, Denborough MA, Foster PS (1995). "Exclusion of defects in the skeletal muscle specific regions of the DHPR alpha 1 subunit as frequent causes of malignant hyperthermia". J. Med. Genet. 32 (11): 913–4. doi:10.1136/jmg.32.11.913. PMC 1051750. PMID 8592342.
- Hogan K, Gregg RG, Powers PA (1996). "The structure of the gene encoding the human skeletal muscle alpha 1 subunit of the dihydropyridine-sensitive L-type calcium channel (CACNL1A3)". Genomics. 31 (3): 392–4. doi:10.1006/geno.1996.0066. PMID 8838325.
- Robinson RL, Monnier N, Wolz W, Jung M, Reis A, Nuernberg G, Curran JL, Monsieurs K, Stieglitz P, Heytens L, Fricker R, van Broeckhoven C, Deufel T, Hopkins PM, Lunardi J, Mueller CR (1997). "A genome wide search for susceptibility loci in three European malignant hyperthermia pedigrees". Hum. Mol. Genet. 6 (6): 953–61. doi:10.1093/hmg/6.6.953. PMID 9175745.
- Monnier N, Procaccio V, Stieglitz P, Lunardi J (1997). "Malignant-hyperthermia susceptibility is associated with a mutation of the alpha 1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle". Am. J. Hum. Genet. 60 (6): 1316–25. doi:10.1086/515454. PMC 1716149. PMID 9199552.
- Meyers MB, Puri TS, Chien AJ, Gao T, Hsu PH, Hosey MM, Fishman GI (1998). "Sorcin associates with the pore-forming subunit of voltage-dependent L-type Ca2+ channels". J. Biol. Chem. 273 (30): 18930–5. doi:10.1074/jbc.273.30.18930. PMID 9668070.
- Morrill JA, Brown RH, Cannon SC (1998). "Gating of the L-type Ca channel in human skeletal myotubes: an activation defect caused by the hypokalemic periodic paralysis mutation R528H". J. Neurosci. 18 (24): 10320–34. doi:10.1523/JNEUROSCI.18-24-10320.1998. PMC 6793372. PMID 9852570.
- Protasi F, Paolini C, Nakai J, Beam KG, Franzini-Armstrong C, Allen PD (2002). "Multiple regions of RyR1 mediate functional and structural interactions with alpha(1S)-dihydropyridine receptors in skeletal muscle". Biophys. J. 83 (6): 3230–44. Bibcode:2002BpJ....83.3230P. doi:10.1016/S0006-3495(02)75325-3. PMC 1302400. PMID 12496092.
- Carsana A, Fortunato G, De Sarno C, Brancadoro V, Salvatore F (2003). "Identification of new polymorphisms in the CACNA1S gene". Clin. Chem. Lab. Med. 41 (1): 20–2. doi:10.1515/CCLM.2003.004. PMID 12636044. S2CID 20811090.
External links
- GeneReviews/NCBI/NIH/UW entry on Malignant Hyperthermia Susceptibility
- CACNA1S+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.


















